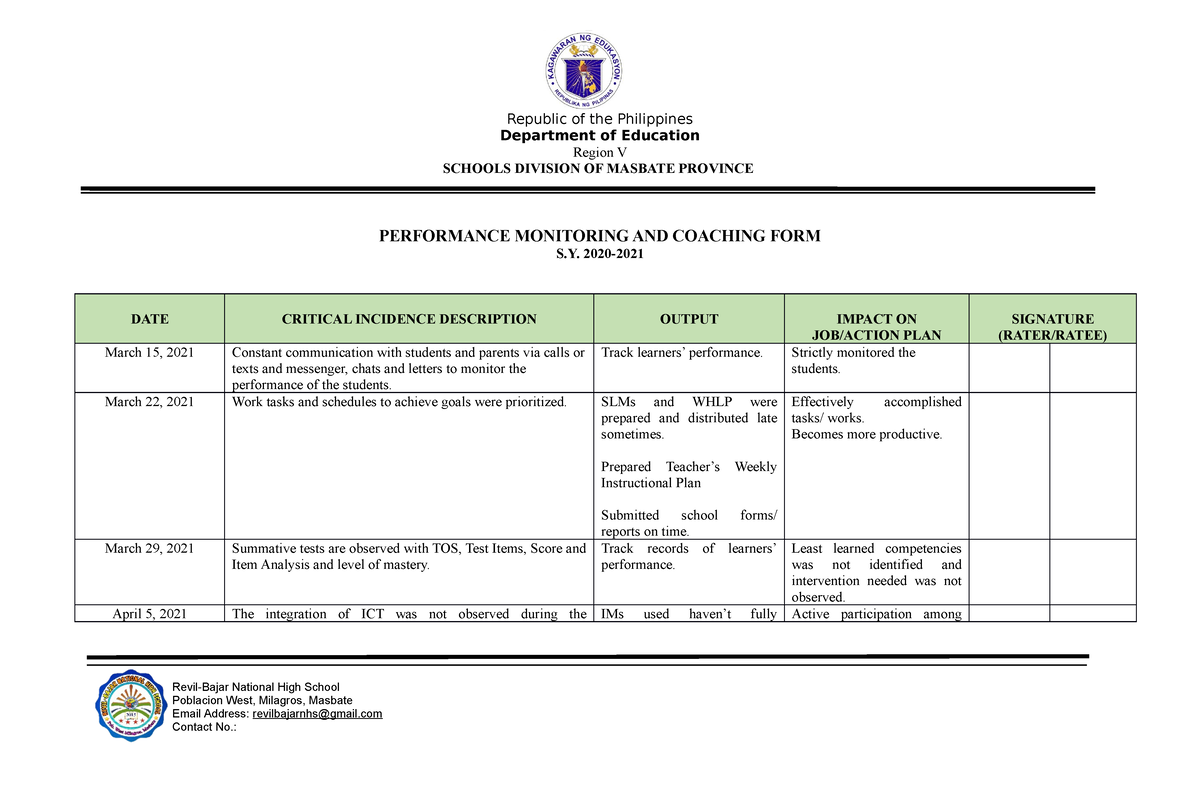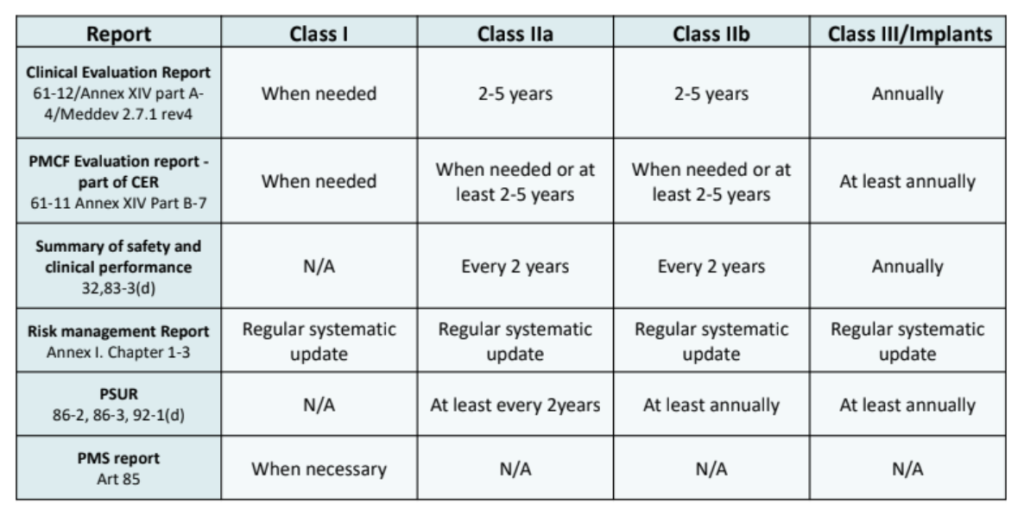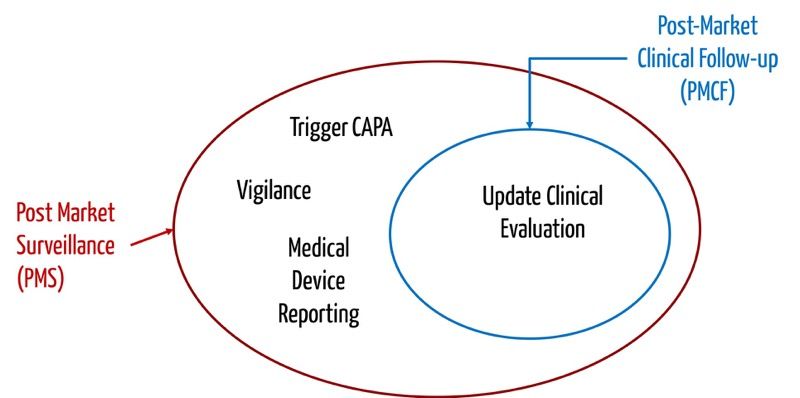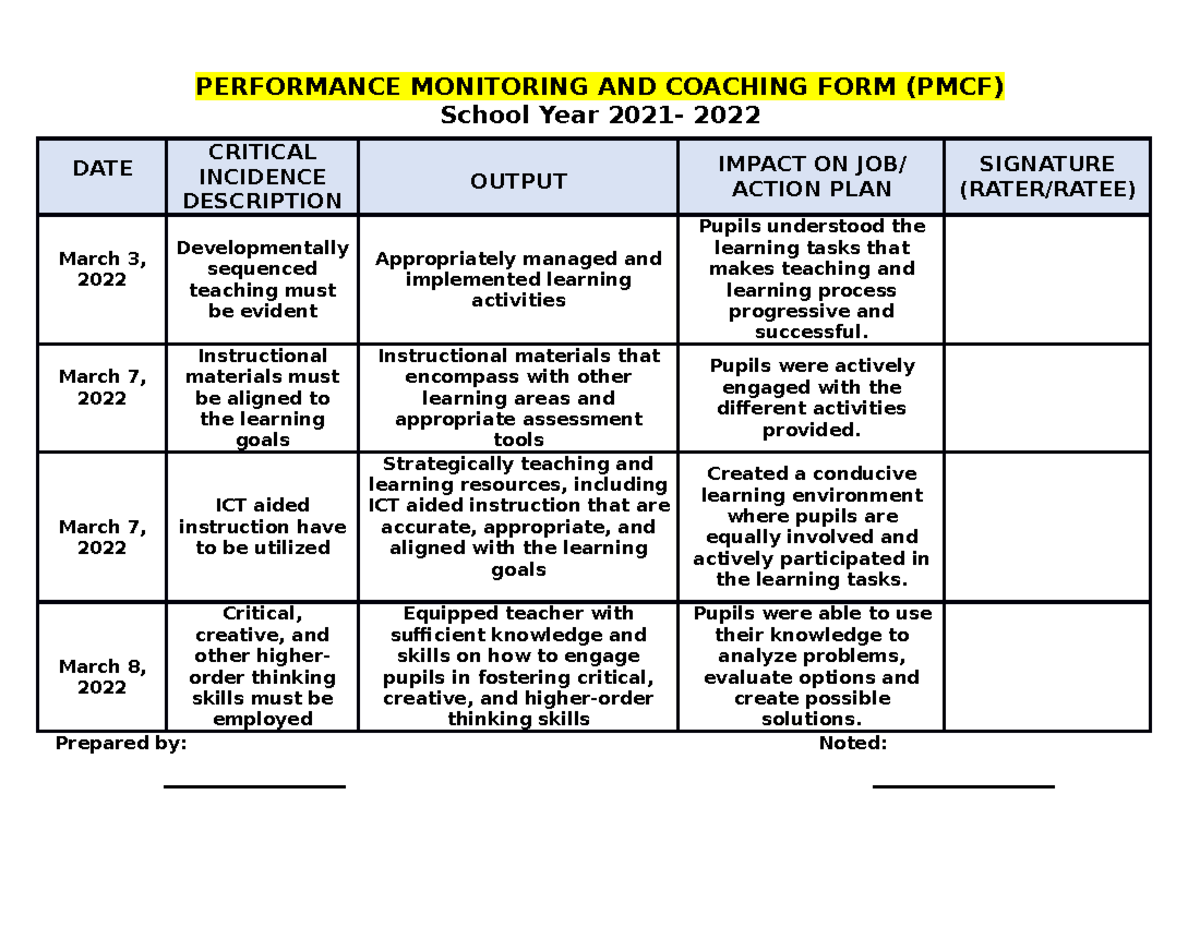
Sample PMCF SY 2021-2022 - School Year 2021- 2022 DATE CRITICAL INCIDENCE DESCRIPTION OUTPUT IMPACT - Studocu
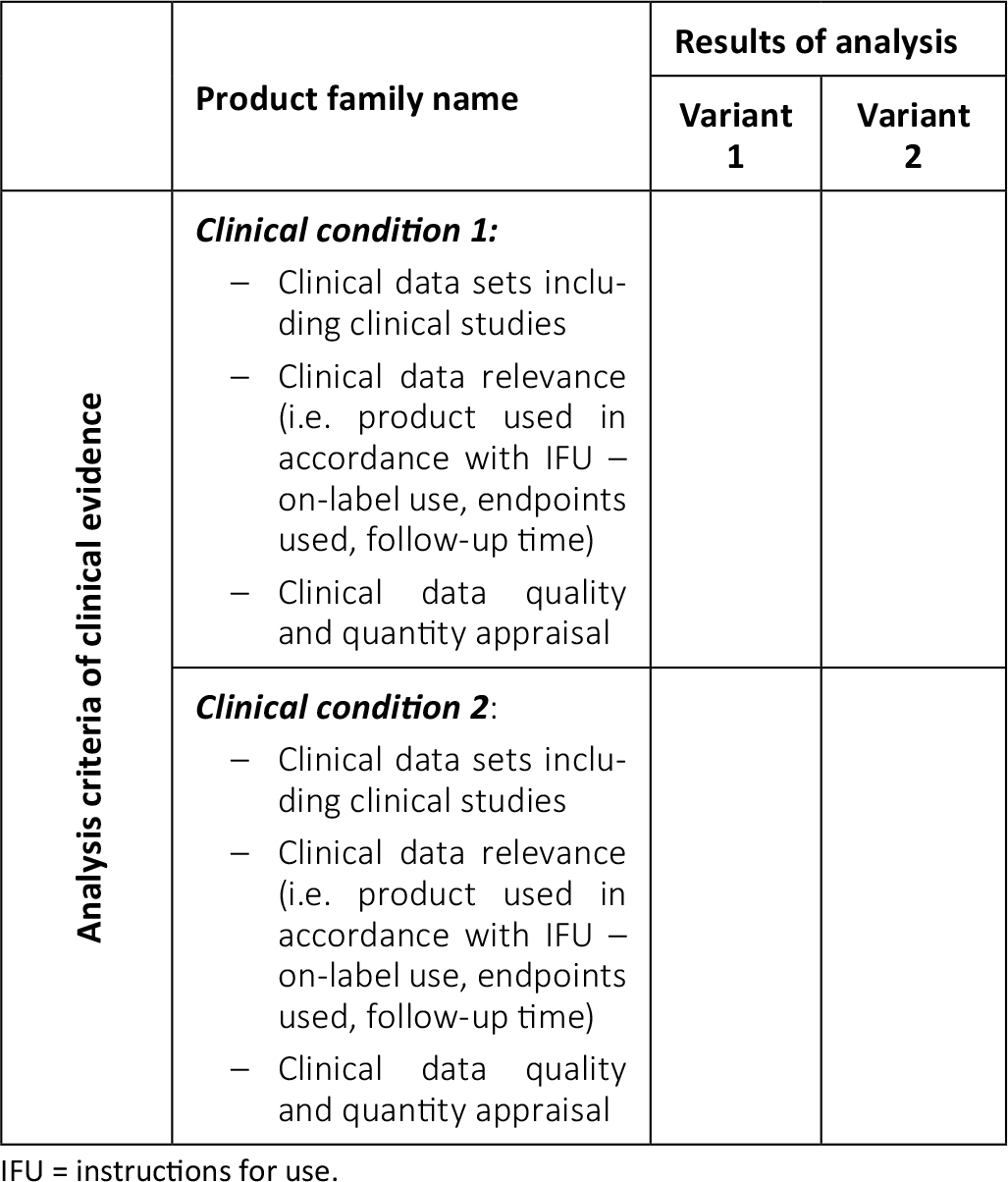
View of Getting your devices ready for MDR compliance – a clinical approach and orthopaedic device manufacturers' perspective | AboutOpen

EU Review and assessment of market surveillance activities 2014-2016 – Medical devices sector – Medical Device Expert News