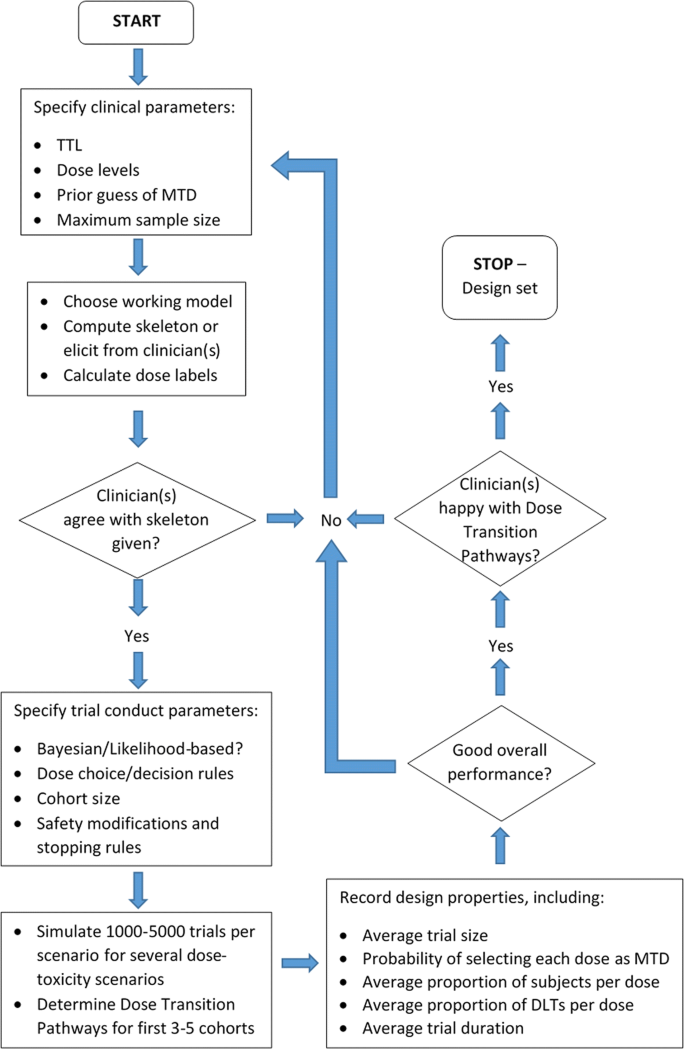
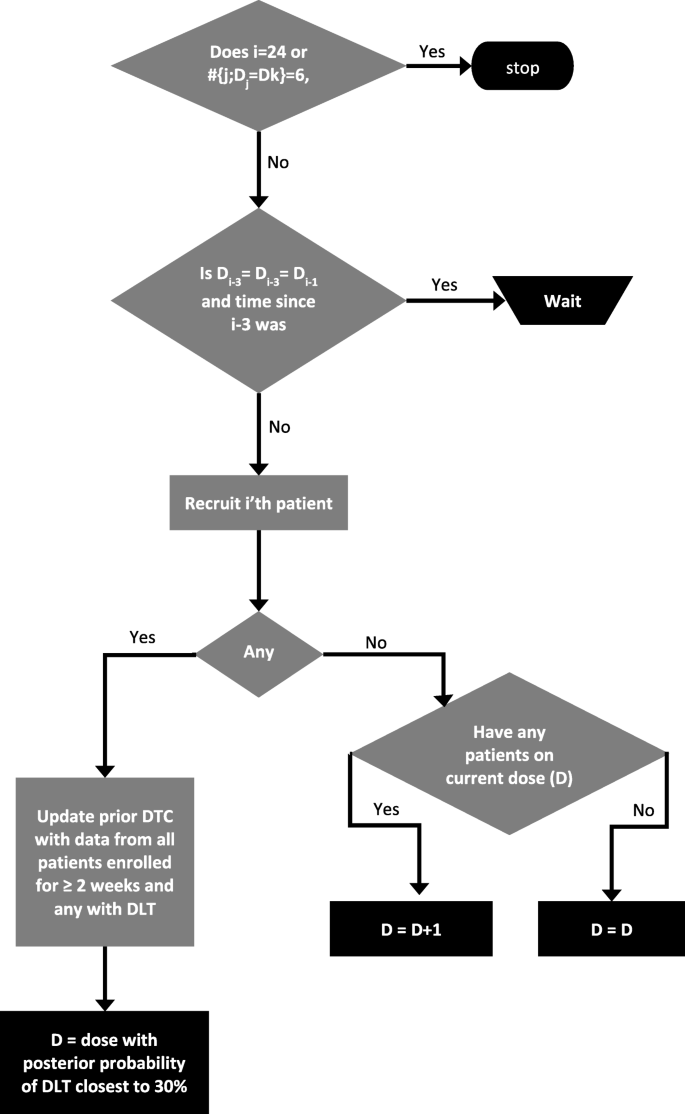
How to design a dose-finding study using the continual reassessment method | BMC Medical Research Methodology | Full Text

Immune checkpoint inhibitor-based combinations: is dose escalation mandatory for phase I trials? - Annals of Oncology
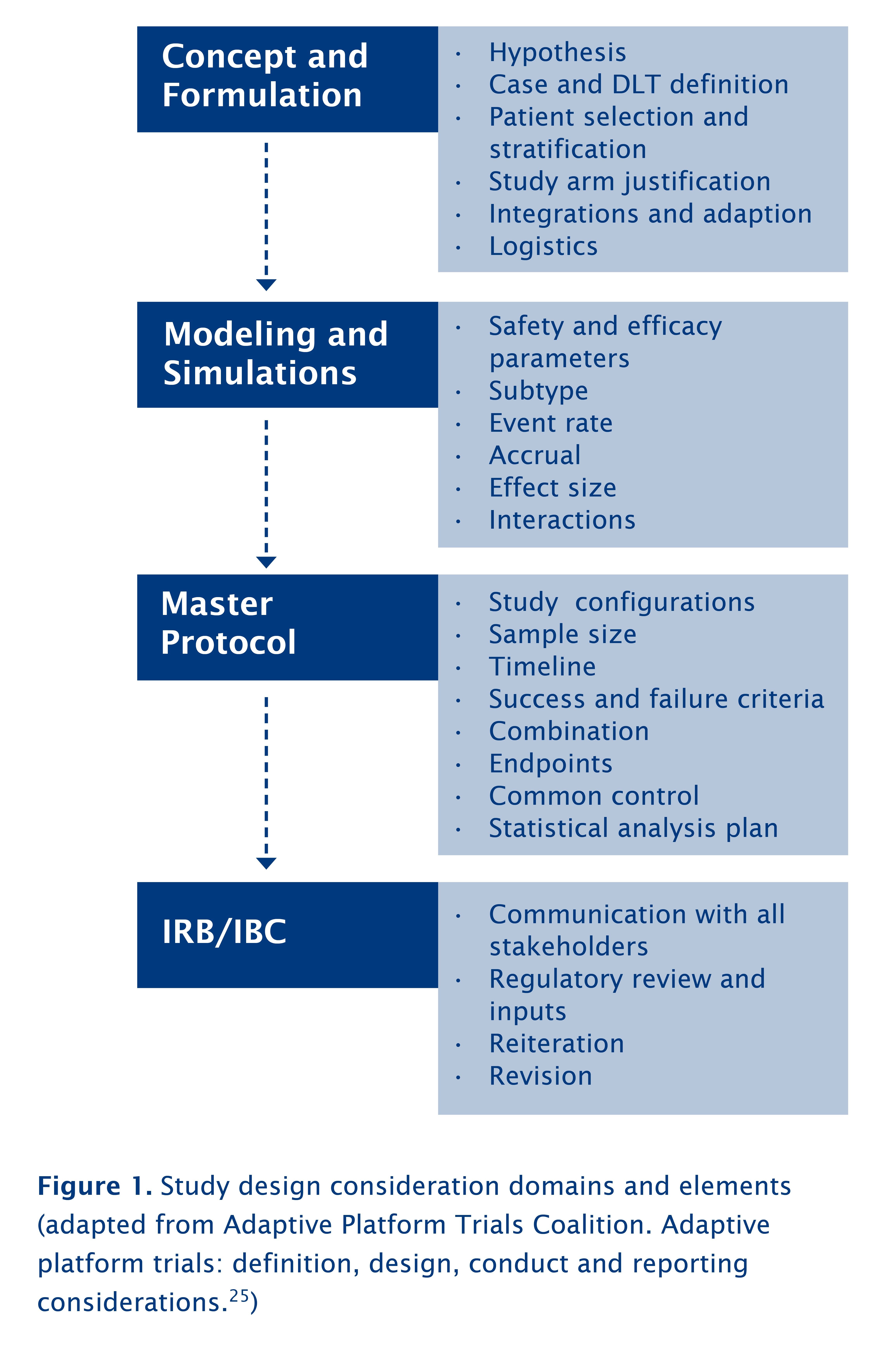
A new pragmatic design for dose escalation in phase 1 clinical trials using an adaptive continual reassessment method | BMC Cancer | Full Text
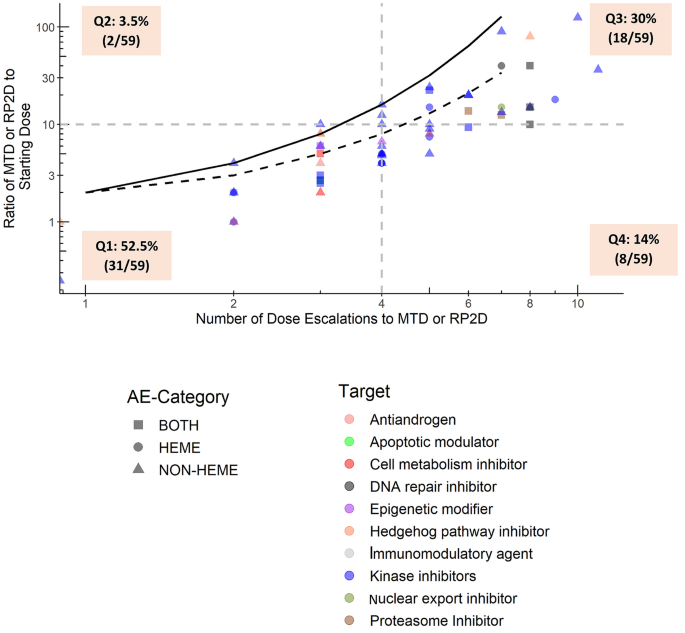
Starting dose selection and dose escalation for oncology small molecule first-in-patient trials: learnings from a survey of FDA-approved drugs | SpringerLink
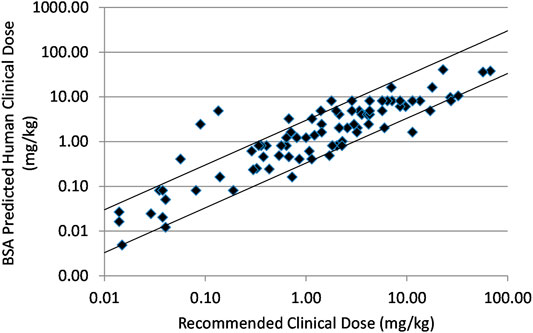
Frontiers | Predicting Approximate Clinically Effective Doses in Oncology Using Preclinical Efficacy and Body Surface Area Conversion: A Retrospective Analysis

Starting dose selection and dose escalation for oncology small molecule first-in-patient trials: learnings from a survey of FDA-approved drugs | SpringerLink
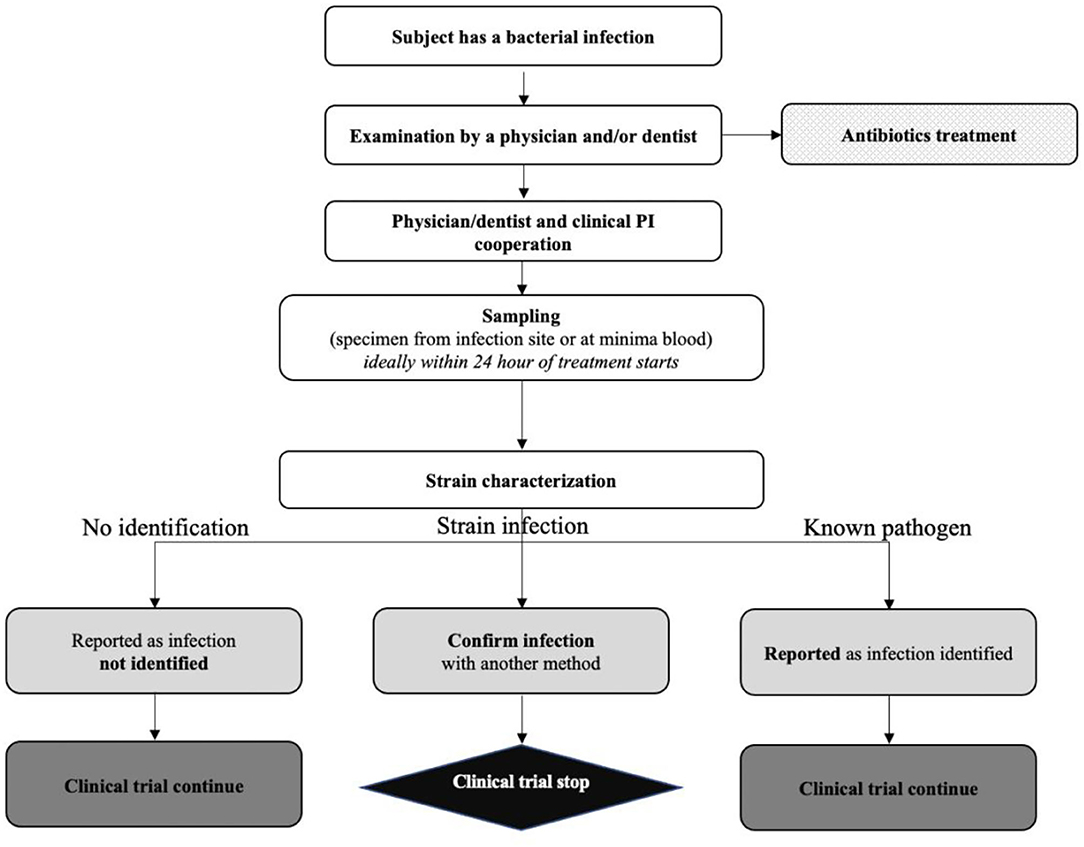
Frontiers | Entering First-in-Human Clinical Study With a Single-Strain Live Biotherapeutic Product: Input and Feedback Gained From the EMA and the FDA

Design and Conduct Considerations for First‐in‐Human Trials - Shen - 2019 - Clinical and Translational Science - Wiley Online Library
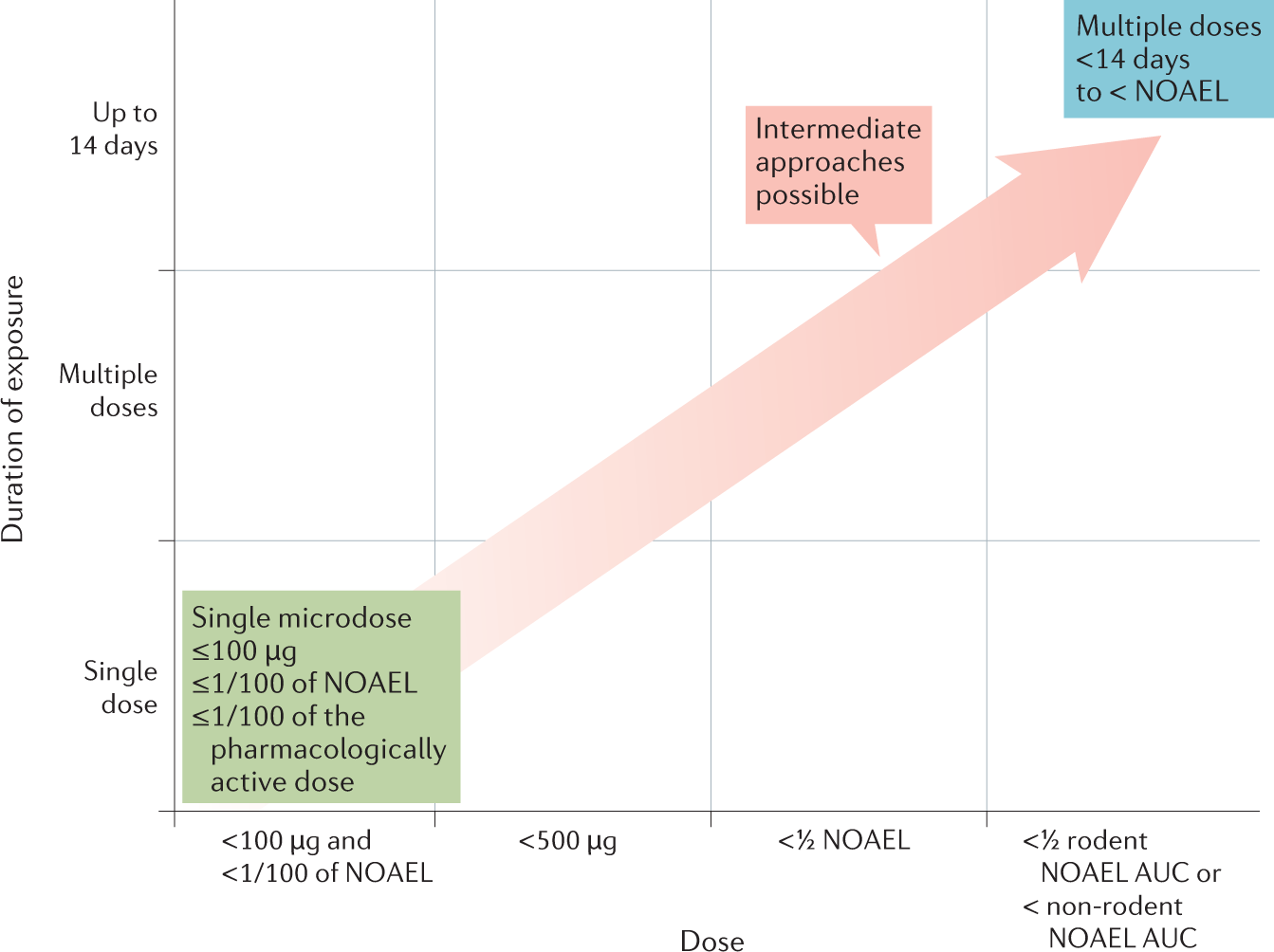
Phase 0/microdosing approaches: time for mainstream application in drug development? | Nature Reviews Drug Discovery

Moving Beyond 3+3: The Future of Clinical Trial Design | American Society of Clinical Oncology Educational Book

Early-drug development in the era of immuno-oncology: are we ready to face the challenges? - Annals of Oncology

Statistical controversies in clinical research: building the bridge to phase II—efficacy estimation in dose-expansion cohorts - Annals of Oncology

FDA draft guidance aims to expedite first-in-human clinical trials for oncology drugs and biologics - Pearl Pathways