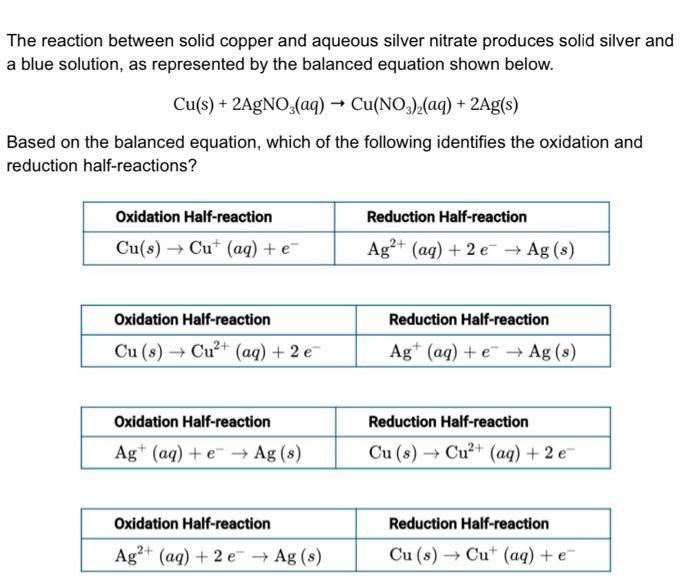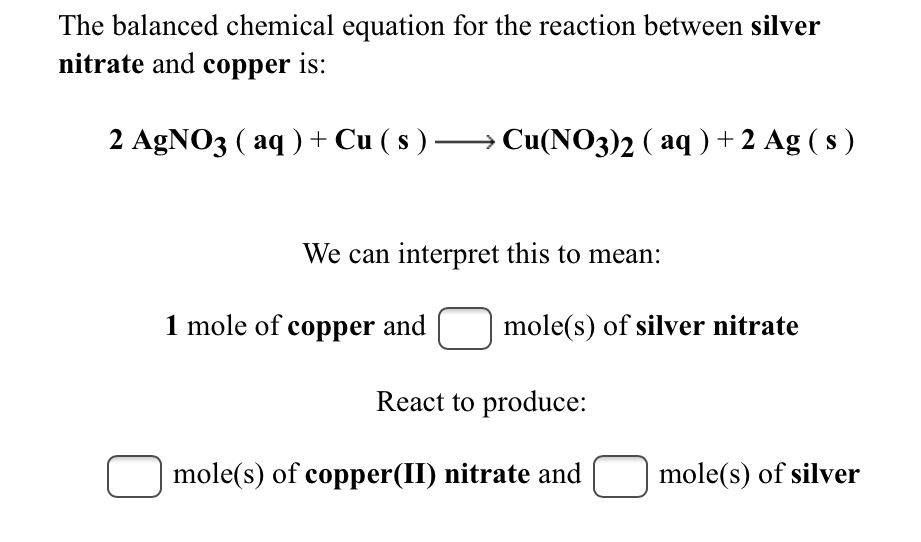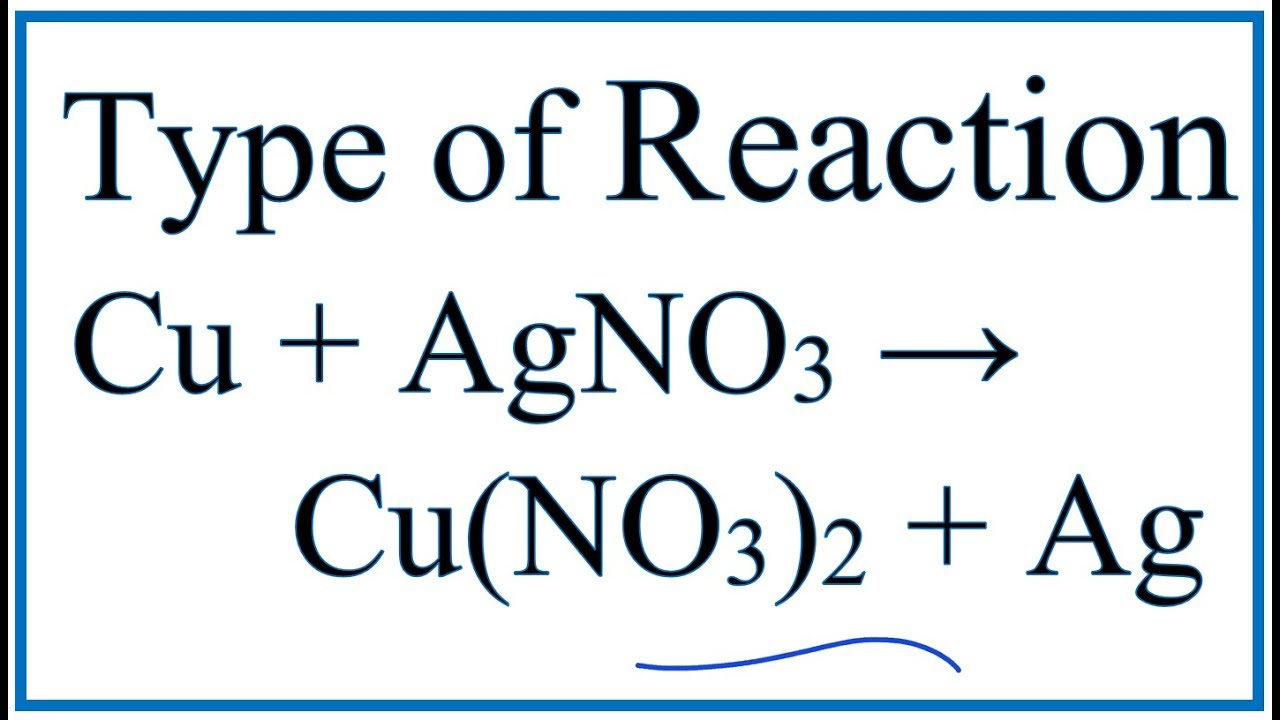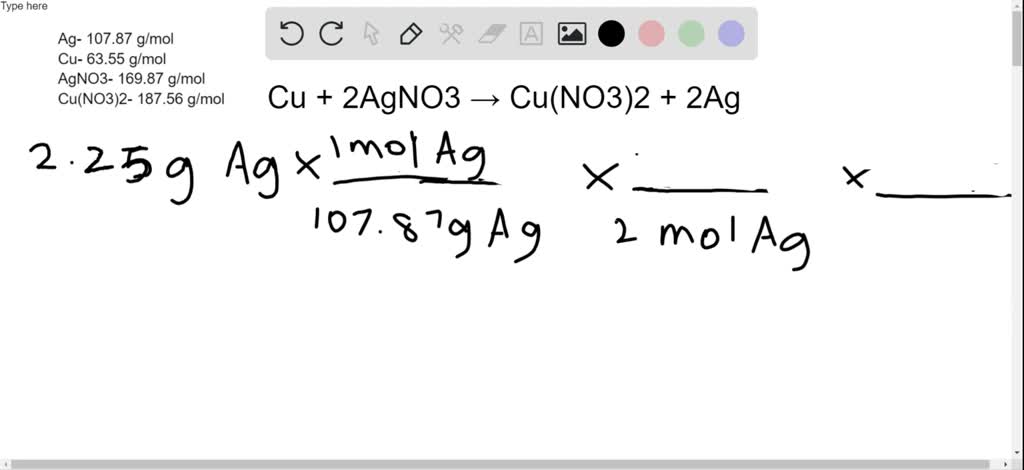
SOLVED:Copper reacts with silver nitrate through single replacement. a. If 2.25 g of silver are produced from the reaction, how many moles of copper(II) nitrate are also produced? b. How many moles
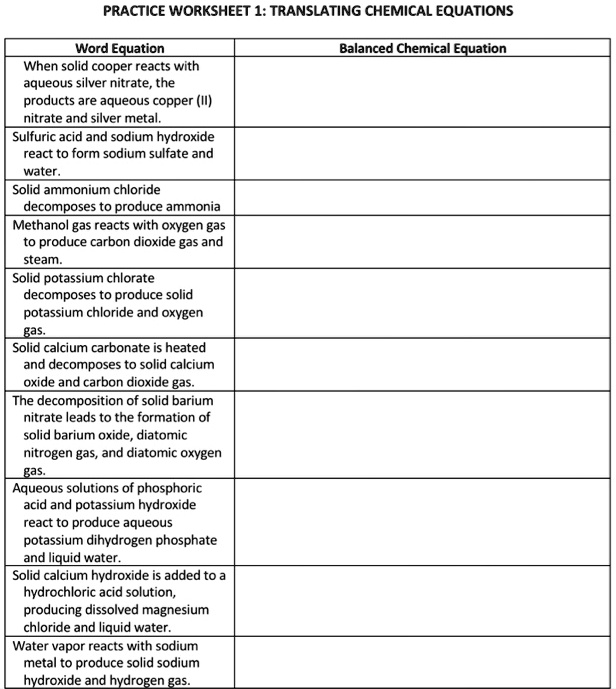
SOLVED: PRACTICE WORKSHEET 1: TRANSLATING CHEMICAL EQUATIONS Word Equation Balanced Chemical Equation When solid cooper reacts with aqueous silver nitrate the products are aqueous copper (II) nitrate and silver metal. Sulfuric acid

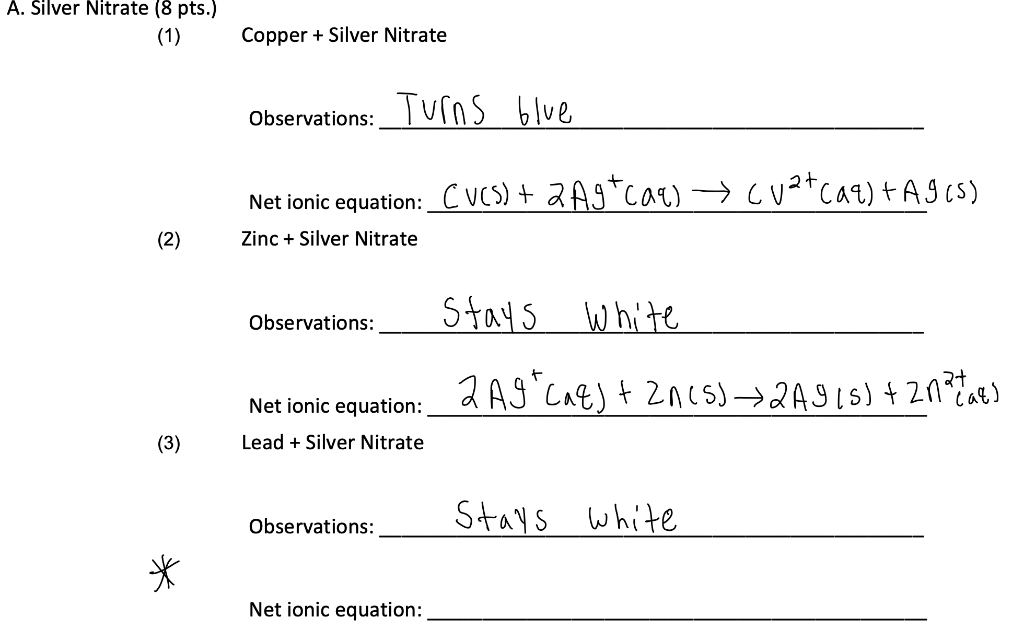
Write balanced chemical equations for these equations : Silver is precipitated out when a copper strip is dipped in silver nitrate solution. The solution turns blue due to the formation of copper (

Conservation of Mass & Word Equations. Demonstration Mass of apparatus and liquids in test tubes Before chemical reaction:______ After chemical. - ppt download
If 34.5 g of copper reacts with 70.2 g of silver nitrate, according to the following reaction, what is the maximum number of grams of silver that can be produced? - Quora

a) The recovery of silver nitrate solution involves displacement by copper metal. Write the - YouTube